Friday, August 30, 2019
Lupine Publishers: Lupine Publishers | The Use of Epidural Anaesthesi...
Lupine Publishers: Lupine Publishers | The Use of Epidural Anaesthesi...: Lupine Publishers | Journal of Veterinary Science Abstract General anaesthesia is an essential component of modern me...
Thursday, August 29, 2019
Lupine Publishers: Change of Evaporations Leads to Climate Change
Lupine Publishers: Change of Evaporations Leads to Climate Change: Lupine Publishers- Environmental and Soil Science Short Communication The basis of all floods and droughts is water, its e...
Tuesday, August 27, 2019
Lupine Publishers: Lupine Publishers| Some Aspects of Red Special Win...
Lupine Publishers: Lupine Publishers| Some Aspects of Red Special Win...: Lupine Publishers- Environmental and Soil Science Abstract Due to increasing environmental radionuclide background, the scien...
Monday, August 26, 2019
Lupine Publishers: Lupine Publishers | Gastrointestinal Parasites Fou...
Lupine Publishers: Lupine Publishers | Gastrointestinal Parasites Fou...: Lupine Publishers | Journal of Veterinary Science Abstract This paper is the first part of a three (3) part series of review...
Saturday, August 24, 2019
Lupine Publishers: Lupine Publishers | Gastrointestinal Parasites Fou...
Lupine Publishers: Lupine Publishers | Gastrointestinal Parasites Fou...: Lupine Publishers | Journal of Veterinary Science Abstract This paper is the first part of a three (3) part series of review...
Lupine Publishers | Updates about Vertebrobasilar Insufficiency in Dizziness
Lupine Publishers | Journal of Otolaryngology
Abstract
Vertebrobasilar insufficiency (VBI) is a result of transitory ischemia of the vertebrobasilar arterial system (VBS) that can produce a variety of symptoms that are on their own are ambiguous. Symptoms include dizziness, vertigo, lightheadedness, headaches, visual changes, diplopia, ataxia, weakness in limbs, pain and stiffness of the neck. Vestibular and visual symptoms can arise suddenly and dissipate rapidly as well, all while preceding more serious symptoms like stroke and death. There are a variety of tests that audiologists and physical therapists can perform as screeners for this impairment, but imaging is an essential component of the diagnosis. Neuroimaging with angiography, magnetic resonance angiography (MRA), magnetic resonance imaging (MRI), and transracial Doppler tests are commonly used. Diagnosis and treating VBI requires a multidisciplinary and interdisciplinary approach. VBI has been documented to be exaggerated and over diagnosed in part due to the vague and transient nature of the symptomology. This paper will further outline the anatomy of the VBS, symptoms of VBI, risk factors, and diagnostic criteria.
Keywords: Atherosclerosis; Balance; Dizziness; Vertebrobasilar Arterial System; Vertebrobasilar Insufficiency
Introduction
The vertebrobasilar arterial system (VBS) supplies the vestibular and cochlear nuclei and is comprised of the vertebral artery (VA), basilar artery (BA), anterior-inferior cerebellar artery (AICA), and the posterior-inferior cerebellar artery (PICA) [1]. The VA and BA supply blood to the pons, medulla, cerebellum, mesencephalon, thalamus, occipital lobes, and of course, the peripheral and central vestibular system, as mentioned above [2]. The reason why changes in the VBS have a greater effect on the vestibular system rather than affecting both cochlear and vestibular function is that the cochlear system also received blood flow from the carotid artery which protects it from suffering the same impairments as the vestibular system which only received blood from the labyrinthine branches of the vertebrobasilar arteries [3]. Furthermore, the ischemia of the VBS can affect both peripheral and central vestibular structures by causing isolated dizziness attacks resulting from ischemia to the vestibular nuclei and/or vestibular cochlear nerve, or directly affecting the labyrinth [3]. The typical cause of hemodynamic changes in this system are the result of atherosclerosis; but other causes are embolism, and arterial dissection, or rarely migraine, fibromuscular dysplasia, and coagulopathies [3]. Considering the anatomy and physiology, it is easy to understand how changes or limitation in blood flow can induce disequilibrium, vertigo, and/or vision changes. Symptoms include dizziness, vertigo, nystagmus, imbalance, lightheadedness, headaches, mental confusion, aural fullness, tinnitus, hearing changes, nausea, vomiting, syncope, diplopia, blurred vision, blindness, ataxia, difficulty swallowing, dysarthria, pain and stiffness in neck or shoulder, and weakness of the extremities [1–4]. While it is possible for isolated and sudden attacks of vertigo to occur, vertigo alone is does not meet the diagnostic criteria for VBI [2,3]. Visual disturbances are commonly congruent with vertigo in VBI patients, but in fact, visual symptoms such as diplopia, visual hallucinations, changes in visual field, and blindness are more common than vertigo [3]. Patient report visual changes, diplopia, palsy of the oculomotor nerve, and seeing spots [2,3]. The onset of symptoms occurs rapidly and can be described as an attack by occurring during the change of position or suddenly reaching maximal affect within 5 minutes of the start and lasting anywhere from 2 minutes to 30 minutes commonly (but has been reported up to 24 hours) [4]. The resolution of the symptoms (or attack) occurs quickly. The frequency and antecedent of symptoms vary making it additionally difficult to diagnose based on patient complaints alone. The incidence is difficult to calculate as it has been recorded that VBI diagnosis has been exaggerated and inappropriately used to diagnose other conditions [5]. There is however a sex effect in that VBI is more likely to be found in men (rather than women) after the fourth decade of life [3].
Discussion
Differential diagnosis of VBI required careful examination to separate the symptoms from other conditions. Recording a detailed case history, performing the appropriate clinical measures, and having the essential imaging is required for adequate differential diagnosis. Case history will be necessary to identify possible risk factors such as: hyperlipidemia, hypertension, cerebrovascular diseases, carotid disease, heart disease, smoking, alcoholism, diabetes, and hyperglycemia [2,5]. Record and description of total symptoms at time of attack and clarifying any vagueness of response or other possible related incidences will be helpful. Also recognize that position changes like moving from laying/sitting to standing can induce symptoms. A vestibular assessment may be useful in differentiating the symptoms from other disorders. In the vestibular assessment the clinician may find that a patient with VBI and vestibular decruitment and hyperactive caloric responses [2,4]. Although hyperactive caloric responses may be observed, the two results of this test, canal paresis and directional preponderance, do not distinguish between an intracranial lesion or a labyrinthine impairment; however, the presence of decruitment/and or hyperactivity is indicative of an impairment as normal patients do not present with these clinical findings [4]. Some patients were also found to have abnormal function in the optokinetic pattern test, eye tracking, or visual suppression test in the videonystagmography (VNG) [1]. Another assessment conducted by the audiologist that may be useful in the Auditory Brainstem Response (ABR) when the ischemic is in the AICA displaying a substantial increase in interpeak latency between waves I-IV and II-IV [1].
A retrospective study done in China by Hu et al. [5] found that out of 773 patients diagnosed with VBI only 67 (8.67%) of them had true VBI. Other conditions to consider are benign paroxysmal positional vertigo (BPPV), Meniere’s disease, vestibular neuronitis, syncope, heart disease, abnormal blood pressure, sudden deafness, infectious diseases, and brain trauma [5]. Accordingly, neuroimaging will be useful in differential diagnosis. Tests include angiography, magnetic resonance angiography (MRA), magnetic resonance imaging (MRI), transracial Doppler, angio-tomography, and cardiologic studies [3,4]. Arteriography is highly useful in diagnosing VBI, but fewer patients are willing to have this test completed due to the risks of arterial catheters, low blood flow, or stroke [4]. The non-invasive test of MRA can be completed to identify possible occlusion or stenosis in neck or intracranial vessels [1,3]. This test has already been useful by identifying that the proximal regions of the vertebral arteries is the location of highest incidence causing VBI, as recorded with MRA [3]. When investigating the basilar artery, the MRA and angio-tomography are found to have similar sensitivity and specificity. The use of transcranial Doppler test is a low-cost, noninvasive, and pain-free test that is used to measure the speed and direction of intracranial arterial blood flow [3]. The plasticity index (PI) recording from the Doppler test is useful in predicting early hemodynamic intracranial variations; however, there is a sex effect in that speech and PI results are opposing for men and women as they age [3]. As evident with the above explanation, there are many useful tests that can be completed to aid in diagnosis of VBI and the need to multidisciplinary and interdisciplinary team is essential.
Conclusion
Understanding risk factors and possible symptoms is helpful in differential diagnosis, however, the risks and symptoms are broad and vague. Patients may present with the exact same symptomology but have varying diagnosis. The use of objective measures, such as caloric testing, and neuroimaging will be vital to accurately diagnosis. Due to the vast symptoms and fluctuating presentations, there is no set diagnostic criteria that can be applied to all patients. This is a concern to protect patients from the progression of the impairment which are stroke and possibly death. Correspondingly, more research needs to be conducted to understand the progression of stenosis as it gradually worsens over time.
For more Lupine Publishers Open Access Journals Please visit our website:
https://lupinepublishers.us/
For more Journal of Otolaryngology-ENT Research articles Please Click Here:
https://lupinepublishers.com/otolaryngology-journal/
https://lupinepublishers.us/
For more Journal of Otolaryngology-ENT Research articles Please Click Here:
https://lupinepublishers.com/otolaryngology-journal/
Friday, August 23, 2019
Lupine Publishers: Lupine Publishers | Use of Dietary Yeast and its P...
Lupine Publishers: Lupine Publishers | Use of Dietary Yeast and its P...: Lupine Publishers | Journal of Veterinary Science Abstract All around the world, sheeps and goats play an important role in ...
Wednesday, August 21, 2019
Lupine Publishers: Major Issues Related to Women Health, Social, Cult...
Lupine Publishers: Major Issues Related to Women Health, Social, Cult...: Journal of Gynaecology | Lupine Publishers Abstract Present article sketches out major issues related to health, social...
Tuesday, August 20, 2019
Lupine Publishers: Bowels and Urine Odors and Its Solutions | Lupine ...
Lupine Publishers: Bowels and Urine Odors and Its Solutions | Lupine ...: Journal of Diabetes and Obesity | Lupine Publishers Opinion From the beginning time people go for bowel outside in the fi...
Thursday, August 15, 2019
Lupine Publishers | Apnea, Hypopnea and Their Individual Effects on Daytime Sleepiness and Sleep Quality
Lupine Publishers | Journal of Otolaryngology
Abstract
Background: Obstructive sleep apnea and hypopnea syndrome (OSA) is defined as a reduction or cessation of the airflow in the human airway. It effects nearly 18 million Americans and weight gain is the main predisposing factor. In this study, we aimed to investigate the effects of apnea and hypopnea individually.
Material and Methods: 83 participants were included in the study and they are divided into two groups as apnea predominant or hypopnea predominant. Pittsburg quality of sleep index (PQSI) and Epworth sleepiness scale (ESS) are completed for all subjects and full-night attended polysomnographic evaluations are done.
Results: ANOVA test was used to compare the inter-group variances. Between the two study groups, no statistical significance was reported between the PSQI or ESS scores.
Conclusion: The effects of apnea and hypopnea are similar on sleep quality or day-time sleepiness, however further studies also investigating the duration of the events as well are needed.
Abbreviations: OSA: Obstructive Sleep Apnea/Hypopnea Syndrome; PSG: Polysomnography; PSQI: Pittsburgh Sleep Quality Index; ESS: Epworth Sleepiness Scale.
Introduction
Obstructive sleep apnea and hypopnea syndrome (OSA) is reviewed under the sleep related breathing disorders. The diagnostic criteria must satisfy daytime sleepiness, fatigue or nonrestorative sleep, waking up with breath holding, gasping or choking, witnessed apnea periods and comorbidities such as hypertension, mood disorder, cognitive dysfunction, coronary artery disease or type 2 diabetes mellitus may accompany the disease. The full-night polisomnography (PSG) must demonstrate at least five obstructive respiratory events (apnea, hypopnea, respiratory effort related arousals) per hour of sleep (apnea/hypopnea index, AHI), but AHI below 15 needs the abovementioned signs for a complete diagnosis [1]. OSA may be seen in any age group, nevertheless published data from several countries indicate that OSA associated with daytime sleepiness occurs in 3% to 7% of adult men and 2% to 5% of adult women. However, because many individuals with OSA do not endorse daytime sleepiness, the prevalence of the disease is likely much higher [2]. The major predisposing factor for OSA is excess body weight. It has been estimated that nearly 60% of moderate to severe OSA is attributable to obesity. The risk of OSA increases as the degree of additional weight increases, with an extremely high prevalence of OSA in people with morbid obesity [3]. Several factors are implicated in the development of OSA [4].
The main cause addresses the reduction of the expansion forces of the dilator muscles of the upper airways. The capacity of the muscles decreases more during the REM sleep. Additional factors are excessive or elongated tissues of the soft palate, macroglossia, tonsillar hypertrophy, and a redundant pharyngeal mucosa [5]. OSA and comorbidities such as stroke, hypertension, metabolic syndrome, cardiovascular diseases or endocrinologic disorders are well taught in years, however the individual effects of apnea or hypopnea alone are never considered. To best of our knowledge, the published data does not mention which entity alone is more harmful to systemic functions or at least sleep, apnea or hypopnea? The Pittsburgh Sleep Quality Index (PSQI) is an effective instrument used to measure the quality of sleep in the adult. It differentiates “poor” from “good” sleep by measuring seven domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction over the last month. A global sum of “5” or greater indicates a “poor” sleeper [6]. The Epworth Sleepiness Scale (ESS) is a self-administered questionnaire with 8 questions. Respondents are asked to rate, on a 4-point scale, their usual chances of dozing off or falling asleep while engaged in eight different activities. The higher the ESS score, the higher that person’s daytime sleepiness and scores higher than 10 are significant. In this study, we compared apnea versus hypopnea due to the sleep quality and daytime sleepiness individually.
Materials and Methods
Between July 2017 and January 2019, 327 cases complaining of snoring, daytime sleepiness and witnessed sleep apnea periods were referred to full-night polisomnography. 150 cases were diagnosed as OSA were enrolled in the study. After the final assessment, a total number of 83 participants were chosen. The ethical committee approval was taken from Okmeydani Training and Research Hospital (48670771–514.10) and informed consent are taken from all participants.
PSG
3 Channel EEG (F4-M1, C4-M1, O2-M1), 2 channel EOG, chin, right and left tibialis anterior EMG, body position sensor, oro-nasal thermal sensor, nasal pressure sensor, thoracic and abdominal sensors, ECG, pulse-oximetry and synchronous video recordings and breath sound recordings were the parameters recorded through the night. The examination, sleep and wake periods and sleep related disorders were scored according to the criteria of the American Academy of Sleep Medicine [7].
Clinical Examination and Laboratory Tests
All participants underwent a detailed otolaryngologic examination including the fiberoptic naso-pharyngolaryngoscopy. Mueller maneuver was made to detect the pharyngeal collapse, vallecula epiglottica was visualized to assess the bulkiness, Friedmann Tongue Positions and Tonsil Gradings are made to determine the glossopharyngeal patency. Complete blood count and routine biochemical blood tests including the thyroid function tests were studied. PSQI and ESS were completed for each participant.
Study Design
In order to emphasize the individual effects of apnea or hypopnea, each PSG were examined and if apnea was higher than hypopnea by 50% or vice versa, that participant was included in the study. The study group, therefore, was divided into two as apnea predominant (AP) and hypopnea predominant (HP). Comorbid pulmonary or neurologic disorders, sleep disorders other that OSA (Central sleep apnea, Hypersomnolence, Parasomnias, Circadian rhythm disorders, etc.) were excluded. Also, pediatric population were not included in the study.
Statistical Analysis
A statistical analysis was performed using IBM SPSS Statistics 22 (IBM SPSS, Turkey). Continuous data was displayed as the mean ± standard deviation. Statistical significance was a p-value of greater than 0.05. A Shapiro–Wilk test showed the normal distribution of the parameters. ANOVA test was used to compare the normally distributed inter-group comparisons of the descriptive statistical methods (mean, standard deviation, and frequency) and the quantitative data.
Results
A total number of 83 participants aged between 24–64 years (mean 46±10 years) were studied. 61 were male (73.49%) and 22 were female (26.51%). The inter-group characteristics are shown in Table 1. The PSQI between AP and HP groups were not statistically significant (p=0.205). Similarly, ESS between AP and HP groups were also not statistically significant (p=0.240) (Figure 1).
Figure 1: AP versus HP by means of PSQI and ESS.
Table 1: Subject characteristics and statistical comparisons.
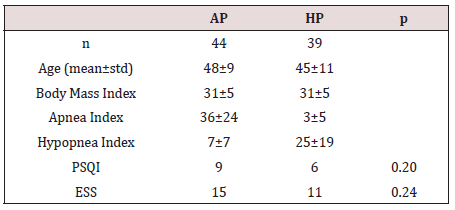
ANOVA test showed no statistical significance among variances.
OSA is defined as the reduction or cessation of airflow for at least ten seconds. The entity is almost every time in association with snoring, and between the snores, airflow may stop completely (apnea) or reduction in the airflow (hypopnea) may happen. If there is a body effort to breathe, the disease is termed obstructive, otherwise it is central. In the presence of a collapsible airway, sleepinduced loss of tonic input to the upper airway dilator muscle motor neurons allows the pharyngeal airway to collapse [8]. The general reaction to this airway obstruction is arousal; sleep then resumes, leading to repeated cycling of sleep, intermittent hypoxia, and arousal throughout the night. Neurocognitive effects of OSA include daytime sleepiness and impaired memory and concentration; cognitive impairment and neural injury may develop in association with sleep apnea [9,10]. Sleep-disordered breathing and OSA are not reported frequently in animals but a natural animal model
Discussion
OSA is defined as the reduction or cessation of airflow for at least ten seconds. The entity is almost every time in association with snoring, and between the snores, airflow may stop completely (apnea) or reduction in the airflow (hypopnea) may happen. If there is a body effort to breathe, the disease is termed obstructive, otherwise it is central. In the presence of a collapsible airway, sleepinduced loss of tonic input to the upper airway dilator muscle motor neurons allows the pharyngeal airway to collapse [8]. The general reaction to this airway obstruction is arousal; sleep then resumes, leading to repeated cycling of sleep, intermittent hypoxia, and arousal throughout the night. Neurocognitive effects of OSA include daytime sleepiness and impaired memory and concentration; cognitive impairment and neural injury may develop in association with sleep apnea [9,10]. Sleep-disordered breathing and OSA are not reported frequently in animals but a natural animal model of OSA is English bulldogs, which have been used to study upper airway anatomy and physiology and the pharmacologic treatment of OSA. English bulldogs have an enlarged soft palate and narrow oropharynx and display many of the clinical features of OSA, including snoring, sleep-disordered breathing, oxyhemoglobin desaturation during sleep, frequent arousal from sleep, and hypersomnolence with shortened sleep latencies [11]. OSA in English bulldogs is not related to obesity, as it often is in humans. OSA has been modeled in a variety of species by using surgical tracheostomy and subsequent intermittent occlusion of the endotracheal tube [12]. Schoorlemmer et al. [13] produced obstructive apnea in conscious rats by using an inflatable balloon implanted in the trachea and apneic episodes of as long as 16 s in duration could be created during sleep. However, animal models of intermittent hypoxemia have several drawbacks. In many cases, the models mimic severe human OSA and may be less applicable to most clinical OSA. In addition, animals exposed to intermittent hypoxemia develop hypocapnia, whereas human OSA is characterized by hypercapnia. Furthermore, human OSA typically is associated with obesity, which is not always considered in animal studies. In addition, OSA causes sleep fragmentation, which may have independent effects on metabolism. Thus, exposure of animals to intermittent hypoxemia produces repeated arousals and changes in sleep architecture that are comparable to those in clinical OSA, yet the effects may not be persistent, limiting their use for studying long-term metabolic consequences of OSA [14].
Animal models are troublesome to study the long-term effects of sleep fragmentation. The sleep quality is a bio-psycho-social parameter that may never be evaluated in animal models; for example, as we refer to excessive daytime sleepiness, the subject is asked whether the sleepiness occurs in the passive state such as resting periods or during the active periods such as work-time or social interactions. Moreover, the sleep architecture, ultradian rhythm or sleep quality may not be assessed in animal models. In our study, the effects of apnea or hypopnea on sleep quality or daytime sleepiness did not differ. This might have several reasons; first of all, all PSG were done elsewhere, our otolaryngology clinic is not capable to perform full-night attended PSG. This situation has some major drawbacks; it is impossible to mark each breath disorders epoch by epoch on the screen of the test computer, but we rather have a brief report of the night. This makes it impossible to calculate the duration of the respiratory events. Therefore, we only could compare the nature of the events by their scores or numbers (Table 1). Secondly, there are some other factors that may interfere with the sleep architecture such as drops in the oxygen levels; not every subject has the same decreasement in their oxyhemoglobine when they have the same level and duration of airway collapse. Thirdly, limb movements also disrupt the sleep quality and impede normal daytime cognitive functions. Finally, arousals are another issue to study; if OSA is recently developed in the subject, the peripheric chemoreceptors detecting the airflow cessation are more sensitive and arousal happens imminently, if the disease is longer the receptors may become insensitive that happens in sleep continuity despite the airflow cessation. Nonetheless, to best of our knowledge, there is no other study that tried to investigate the effects of apnea of hypopnea individually on sleep quality or daytime sleepiness.
Conclusion
The effects of apnea and hypopnea are similar on sleep quality or daytime sleepiness, however further studies also investigating the duration of the events as well are needed.
For more Lupine Publishers Open Access Journals Please visit our website: h
https://lupinepublishersgroup.com/
For more Journal of Otolaryngology-ENT Research articles Please Click Here:
https://lupinepublishers.com/otolaryngology-journal/
https://lupinepublishersgroup.com/
For more Journal of Otolaryngology-ENT Research articles Please Click Here:
https://lupinepublishers.com/otolaryngology-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers
Wednesday, August 14, 2019
Lupine Publishers: Lupine Publishers - Quora
Lupine Publishers: Lupine Publishers - Quora: Lupine Publishers is a premier, peer-reviewed, multidisciplinary, open access, scientific Publisher that aims to publish original work of im...
Tuesday, August 13, 2019
Lupine Publishers | Aging, Hearing Loss and Tinnitus
Lupine Publishers | Journal of Otolaryngology
Abstract
Introduction
However, some physiological changes that happen with the advancing age will only manifest itself from the third age. For example, the human ear reaches maturity around 18-20 years and from this age the hearing organ begins to age, either by loss of sensory cells, neurological degeneration, exposure to ototoxic agents or noise [3]. Hearing loss in the elderly, also known as presbycusis, is a bilateral hearing loss for high frequency sounds. The main clinical manifestations of presbycusis include symmetrical and slowly developing sensorineural hearing loss, high pitched tinnitus and speech recognition disorders [4]. In the case of presbycusis, generally, the hearing thresholds increase significantly between 70 and 80 years of age and reach another stable stage at high levels after 80 years of age, especially in high frequencies [5]. Tinnitus is a common complaint defined as a sound in the head or ears that occurs in the absence of any external acoustical source [6]. It may be caused by several conditions: otological, metabolic, neurological, orthopedic, cardiovascular, pharmacological, odontological and psychological, which in turn may be present concomitantly in the same individual [7]. It affects about 15% of the world population. It can occur at any stage of life, but the highest prevalence occurs in the elderly, probably due to deterioration of the auditory and vestibular systems [8,9]. Tinnitus is the second most common otorhinolaryngological complaint in the elderly [10], with tinnitus often more disturbing than hearing loss [11]. Approximately 33% of the elderly population is affected by tinnitus and 15% to 25% of them present interference with the quality of life caused by this symptom [12]. Both hearing loss and tinnitus can trigger important communication problems, which in turn lead to difficulties in social, occupational and family adaptation. It is very common for elderly individuals to report that they can hear but not understand speech. Some studies have attempted to identify the relationship between age, gender, hearing loss and tinnitus [13-15]; however, which not confirm that tinnitus discomfort could be explained by age, gender, and hearing loss. The hearing loss might be the most dangerous factor and if the its serious, the incidence of the tinnitus became higher. So, tinnitus in the elderly may be the result of a combination of factors. Therefore, other issues are likely to be investigated, such as psychological issues or underlying diseases. To date, is known that exist a high prevalence of hearing loss and tinnitus in the elderly, and that these have a high impact on the patient’s quality of life. Becoming a factor of great negative repercussion for this population, hindering sleep, social life, concentration on daily and professional activities. The first step in care is to investigate the patient’s history. A detailed anamnesis, which should address, in addition to questions about tinnitus, associated diseases, patient’s lifestyle, diet, genetics, general health and the current effects of the disease on the patient’s life. In addition to the anamnesis, the use of questionnaires is important in the evaluation of individuals with tinnitus, as it helps to confirm the presence of tinnitus and determines the severity of the symptoms [16,17]. Treatment may be based on direct reduction of severity or elimination of tinnitus, such as working with the patient’s emotions in the face of tinnitus [18]. Other therapies also known are relaxation techniques, cognitive-behavioral therapy (CBT), psychological counseling, sound therapy, including hearing aids or sound generators, or a combination of these approaches [19]. Until now, CBT is the oneoff have scientific evidence for tinnitus treatment. However, when the patient has hearing loss and tinnitus, the Tinnitus Retraining Therapy has been demonstrating that this model of intervention becomes a treatment option for the relief of tinnitus in the elderly people [20] and of hearing aids could reduce the perception of tinnitus sound intensity and the bothersome with this symptom and with hearing loss [21].
Conclusion
For more Lupine Publishers Open Access Journals Please visit our website: h
https://lupinepublishersgroup.com/
For more Journal of Otolaryngology-ENT Research articles Please Click Here:
https://lupinepublishers.com/otolaryngology-journal/
https://lupinepublishersgroup.com/
For more Journal of Otolaryngology-ENT Research articles Please Click Here:
https://lupinepublishers.com/otolaryngology-journal/
Wednesday, August 7, 2019
Lupine Publishers: Metals Phytotoxicity Assessment and Phyto Maximum ...
Lupine Publishers: Metals Phytotoxicity Assessment and Phyto Maximum ...: Journal of Chemical Sciences | Lupine Publishers Abstract In this paper, the influence of metals (Cd, Pb, Cu, Co, Ni, Z...
Friday, August 2, 2019
Lupine Publishers: Lupine Publishers | Survey on Pathological Lesion ...
Lupine Publishers: Lupine Publishers | Survey on Pathological Lesion ...: Lupine Publishers | Journal of Veterinary Science Abstract A cross-sectional study was conducted from October 2016 to July...
Subscribe to:
Comments (Atom)
Choanal Atresia Repair, A Comparison Between Transnasal Puncture With Dilatation And Stentless Endoscopic Transnasal Drilling
Abstract Background: in this study we present the outcome of surgical repair of choanal atresia of 33 patients underwent t...
